Sustainability
We are a clinical-stage biotechnology company focused on enhancing the health and well-being of individuals living with serious viral diseases. This commitment drives our efforts to forge new and differentiated paths to treating these conditions, inspired by the needs of these individuals.We are a growing team of biotechnology professionals based in corporate offices and laboratory space in the United States. All of our product candidates are currently in clinical development or in varying stages of pre-clinical development.
We focus our corporate responsibility programs on those issues that support the long-term sustainability of our operations and the management of relevant environmental, social, and governance (ESG) risks. Those programs, and relevant disclosures, are aligned with the following topics identified for the biotechnology and pharmaceutical industry by the Sustainability Accounting Standards Board (SASB):
Business ethics
We are committed to conducting our business with integrity and in compliance with all laws, rules, regulations, and corporate policies that apply to our business. In particular, we are committed to acting responsibly, safely, and with transparency in our interactions with all stakeholders in the healthcare system.
Our Code of Conduct outlines our expectations for how employees should handle interactions, transactions and business opportunities.
Compliance programs
Our corporate compliance program is designed to help maintain the highest professional and ethical standards.
Assembly Bio’s General Counsel, as the “Designated Officer” under the Assembly Bio Code of Conduct, and Assembly Bio’s Legal team ensure that periodic reviews and trainings are performed across our organization to assess compliance with our Code of Conduct and all related ethics policies.
We maintain and implement formal risk assessment procedures for all intermediaries, consultants, and suppliers, and annually monitor and report on HR and compliance matters. We use a globally recognized, third-party partner to provide external assurance of compliance with our ethical standards, a minimum of once a year.
All Assembly Bio employees receive training on the Code of Conduct when they join the company. We expect our employees to read, understand, and abide by the requirements in the code to ensure ethical business practices and compliance throughout our organization.
Assembly Bio has a complete program of compliance training on a wide variety of topics. All new employees receive training on anti-bribery and anti-corruption, conflicts of interest, anti-harassment, cybersecurity, and other policies and procedures that outline how they are expected to conduct their day-to-day responsibilities.
Reporting a concern
Employees who have any concerns regarding company ethics are encouraged to express concerns in accordance with the procedures described in the Code of Conduct and the Whistleblowing and Whistleblower Protection Policy.
Assembly Bio has established various ways for individuals to raise matters of concern regarding the conduct of company officials, or ethical, legal, or other issues related to the way the company is conducting its business. One avenue for raising such issues is the Assembly Bio Hotline.
The Assembly Bio Hotline is available to report any conduct or action that is or may appear inconsistent with applicable law, Assembly Bio policies, or the Assembly Bio Code of Conduct. The Hotline provides a means for employees, contractors, and third parties to file a report anonymously (where permitted by applicable law) 24 hours a day, seven days a week, to a third-party service provider that will ensure a caller’s confidentiality.
Any reported allegations of retaliation will be investigated promptly. The investigation may include individual interviews with the parties involved and, when necessary, with individuals who may have observed the alleged conduct or may have other relevant knowledge.
Confidentiality will be maintained throughout the investigation process to the greatest extent possible consistent with adequate investigation and appropriate corrective action. Assembly Bio does not tolerate retaliation of any kind against an employee who reports in good faith a violation of law or of our policy.
Clinical trial programs and standards
We use Good Clinical Practice (GCP) for designing, conducting, recording, and reporting on our clinical trials. GCP is an international ethical and scientific quality standard that is provided by the International Council on Harmonization (ICH). Compliance with this standard provides the public assurance that the rights, safety, and well-being of trial participants are protected and that the clinical trial data are credible.
- REC-11534 (Food and Drug Administration (FDA). (01Apr2017). 21 CFR 50. Protection of Human Subjects. pp. 340-352.)
- EC-12035 (DHHS FDA CDER CBER ICH. (2018). Guidance for Industry – E6 (R2) Good Clinical Practice: Integrated Addendum to ICH E6 (R1))
- Any other country specific regulations which may be applicable to the conduct of the clinical study.
Consent forms provide information regarding the rights of trial subjects and relevant contact information in the event of a concern or complaint.
All employees in our Clinical Operations team are required to attend Good Clinical Practice training.
- Replacement: using alternative non-animal systems in place of live animal utilization whenever possible;
- Reduction: using the minimum number of animals possible to achieve maximum information without compromising animal welfare; and
- Refinement: improving procedures to limit the discomfort and distress to animals undergoing animal studies.
Access to healthcare
Human capital
We are a team of biotechnology professionals, most of whom live and work only in the US.
Attracting the best talent
At Assembly Bio our employees are committed to improving the lives and health of people living with serious viral diseases every day. As a company, we are committed to our employees and their families’ health and wellness, through a strong program of benefits that place them at the center. We believe that first-in-class benefits support and retain first-in-class employees.
- Medical, dental, and vision care coverage (premiums for all Assembly Bio medical, dental and vision plans are 100% covered by the company for employees and their enrolled family members)
- Life, long-term care, disability, and accident insurance and critical illness benefits
- Health care flexible spending account
- Dependent care flexible spending account
- 401k with matching funds
- Employee Stock Purchase Plan (ESPP)
- Paid family leave (up to six weeks at 100% of the employee’s base pay to bond with a new child entering the family through birth, adoption, or foster care placement, or to care for the employee’s seriously ill family member or the equivalent of a family member)
- Paid time off (including holidays, vacation time, and sick time)
- Flexible, non-accrual vacation policy
- Educational assistance program
- Mental health and wellness programs
- Employee assistance program providing digital coaching and clinical level support to employees and dependents on a variety of topics
- Employee referral awards
- Peer-to-Peer recognition awards
- Business travel assistance
- Fitness reimbursement program
- Onsite gym (Headquarters)
- Commuter transit and parking benefit (Headquarters)
- Cell phone reimbursement program
- Financial and wealth management seminars
- Pet insurance at a discounted group rate
- Care support, administrative and logistical, for employees and their families
Training and development
At Assembly Bio, we recognize that today’s employees expect opportunities to learn and grow. We evaluate training and development needs and seek to offer solutions that are aligned with employee needs and our business strategy. We believe that a rich training and development curriculum satisfies two key business objectives: (1) increasing employee engagement through ongoing career development and (2) driving positive outcomes in our clinical stage programs.
Assembly Bio remains committed to building an increasingly diverse and talented work team. As a talent developer, we recognize the criticality of various modes of continuous learning to keep up with the urgency and pace of the biotechnology field today. We have partnered with Kantola Training Solutions to provide our employees with a thorough online training curriculum. Kantola is one of the largest online training companies, having been awarded the Summit International’s “Leader” Award for Emerging Media. We believe that live training and online training, when combined, offer varied learning opportunities and significantly increase our training topics.
- Cyber Security
- Anti-Harassment
- Time Management for Supervisors
- Conflicts in the Workplace
- Creative Confidence
- The Well-Managed Meeting
- Management & Leadership Skills
- Performance Coaching
- Leadership at Every Level
- Leadership for Innovation
- Employment Laws: What Supervisors Need to Know
- Workplace Ethics: Code of Conduct Training for Employees
- Preventing and Managing Stress
- Listening Under Pressure
- Professional Email Etiquette
We believe in supporting our employees’ efforts to deepen their professional knowledge, update their technical skills, and grow their careers. The Assembly Bio Educational Assistance Program reimburses employees for tuition and textbook costs up to a maximum per year for courses that directly enhance their areas of professional work or contribute to their immediate career growth. The Educational Assistance Program is an important investment in our employees that also reaffirms our commitment to analytical/conceptual growth, enhanced knowledge, and professional development. All full-time employees who have been employed in a full-time position for six months are eligible for the program.
Diversity and inclusion
Assembly Bio is committed to fostering, cultivating, and preserving a culture of diversity and inclusion. The collective sum of the individual differences, life experiences, knowledge, inventiveness, innovation, self-expression, unique capabilities, and talent that our employees invest in their work represents a significant part of not only our culture, but our company’s achievement as well. At Assembly Bio, everyone has a seat at the table.
Assembly Bio diversity data
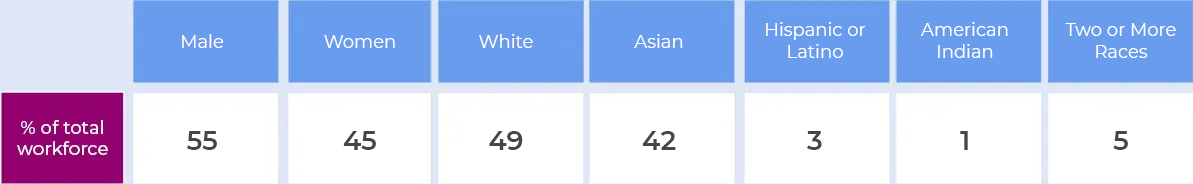
Initiatives to promote diversity and inclusion in our business include:
- Completion of a pay equity study that confirmed no statistically significant gender or ethnic pay gap
- Our job descriptions are designed to encourage a diverse range of applicants and encourage any and all applicants of all genders and races even if they feel they are only partially qualified for the applicable role
- Membership in the NOBCChE’s LinkedIn group (National Organization for the professional advancement of Black Chemists and Chemical Engineers) and the NIH Women of Color Research (WoCRn) LinkedIn group
- Completion of the Biocom Diversity, Equality and Inclusion (DEI) Survey, the results of which will inform us regarding best DEI practices in the biotechnology industry
- Posting new positions on pharmadiversity.com and The Mom Project
Environment, health
and safety
Our commitment to EHS is confirmed in our company wide EHS policy.
Environment
Our impact on the environment matters to us and to our stakeholders. We are committed to doing our part to support the shift to a low-carbon economy and to lessen the impacts of climate change. We have implemented energy efficient lighting in our offices and continue to seek ways to further reduce our environmental footprint.
All employees are encouraged to conserve resources and reduce waste and emissions through composting and recycling programs and other energy conservation measures.
Health and safety
Assembly Bio is committed to providing a safe and healthy work environment for all employees and anyone else working on our premises.
Our laboratory safety policies, designed to provide employees with clear standards of work practices and procedures to decrease health and safety risks, are described in our comprehensive Laboratory Safety Manual. Employees and guests are expected to follow all applicable practices and procedures contained in the Manual and to report any hazardous and unsafe conditions to the Safety Committee.
The Manual and its policies also identify the minimum training requirements that must be completed and documented before an employee begins any work in the laboratories. The extent of the safety training will depend on the type of work the employee will be conducting and, based on need, additional training will be provided for specific hazardous work.
The Laboratory Safety Manual is accompanied by other manuals that contain further explanations of various areas of safety.
- Chemical Hygiene Plan
- Bloodborne Pathogens Exposure Control Plan
- Emergency Action Plan
- Injury & Illness Prevention Plan (IIPP)
- Fire Prevention Plan
Laboratory personnel must have access to the Manual at all times.
Chemical safety and hazardous waste management
Assembly Bio has developed and implemented a Chemical Hygiene Plan (CHP) which specifies prudent practices associated with work involving hazardous chemicals in the Assembly Bio labs. The CEO is ultimately responsible for chemical hygiene within Assembly Bio and must, with other administrators, provide continuing support to achieve compliance with the CHP.
The CHP includes procedures for:
- Laboratory facility design and maintenance
- Chemical procurement, distribution, storage and monitoring
- Monitoring employee exposure
- Protective equipment and systems
- Hazardous waste disposal
Emergency preparedness
Assembly Bio has developed and maintains a dedicated Emergency Action Plan, which outlines the steps and guidelines for handling any site emergency, including:
- Chemical spills
- Biohazard spills and exposure
- Fire
- Power outage
- Flooding
- Evacuation



